Technology-driven catalysis
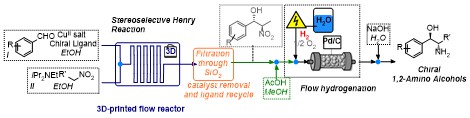
Stereoselective catalytic APIs synthesis in home-made 3D-printed mesoreactors
S. Rossi, D. Brenna, R. Porta, A. Puglisi, M. Benaglia Angew. Chem. Int. Ed., 2017, 56, 4290-4294. [Link]
Abstract:
3D-printed flow reactors were designed, fabricated from different materials (PLA, HIPS, NYLON),
and used in a catalytic stereoselective Henry reaction. The use of readily prepared and tuneable 3D-printed reactors allowed
for a rapid screening of devices with different sizes, shapes and channel dimensions, aimed at the identification of the best
performing reactor set up. The optimized process afforded the products in high yields, moderate diastereoselectivity and up
to 90% e.e.. The method was applied to the synthesis in flow of chiral 1,2-amino alcohols displaying biological activities
(norephedrine, metaraminol, and methoxamine), through a two-steps, all-in-flow sequence, that involves, after the nitroaldol
reaction, a continuous flow hydrogenation. To highlight the potential industrial application of this methodology, a multistep
continuous synthesis of norephedrine has been realized: the product was isolated without any intermediates purification or
solvent switching operation.
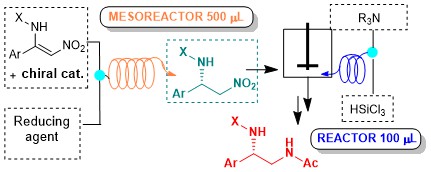
A continuous flow, two-steps, metal-free process for the synthesis of differently substituted chiral 1,2-diamino derivatives
M. Pirola, M. Benaglia, M. E. Compostella, L. Raimondi, A. Puglisi Synthesis, 2018, 50, 1430-1438 [Link]
Abstract:
The enantioselective organocatalytic reduction of aryl-substituted nitroenamines was successfully performed under
continuous-flow conditions. After a preliminary screening with a 10 ul microreactor, to establish the best reaction conditions, the reduction
was scaled-up in a 0.5 ml mesoreactor, without appreciable loss of enantioselectivity, that remained constantly higher than 90%. The in-flow
nitro reduction was also accomplished, either by Raney-Ni catalyzed hydrogenation, and by a metal-free methodology based on the
use of the. very inexpensive and readily available reducing agent trichlorosilane. The final aim is to develop a two-step,
continuous-flow process for the stereoselective, metal-free, catalytic synthesis of differently functionalized chiral 1,2-diamines
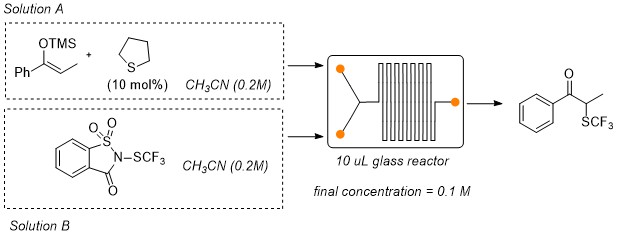
Organocatalytic α-trifluoromethylthiolation of silylenol ethers: batch vs continuous flow reactions
S. S. Abubakar, M. Benaglia, S. Rossi, R. Annunziata Cat. Today, 2018, 94-101 [Link]
Abstract:
This work describes the organocatalytic a-trifluoromethylthiolation of silylenol ethers using N-(trifluoromethylthio)saccharin as
trifluoromethylthiolating reagent that is activated by the presence of catalytic amounts of a Lewis base. Tetrahydrothiophene was identified
as the best organocatalyst and it was successfully employed to promote the synthesis of different α-trifluoromethylketones; the reaction
has been performed under a traditional batch methodology and under continuous flow conditions. In general, yields obtained using the traditional
batch process were higher than those observed when the reaction was performed under flow conditions. However, short reaction times, higher
productivity and higher space time yields were observed when a flow system process was employed. Preliminary DFT calculations were also
performed in order to elucidate the mechanism of the reaction.
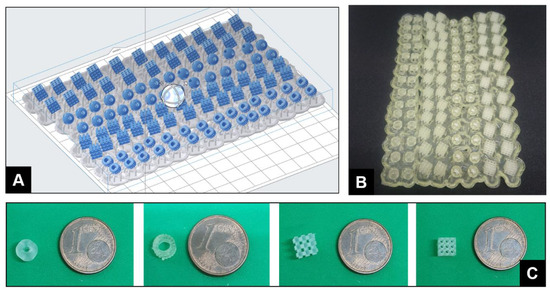
Stereolithography 3D-Printed Catalytically Active Devices in Organic Synthesis
S. Rossi, A. Puglisi, L. Raimondi, M. Benaglia, Catalysts 2020, 109, 1-9. [Link]
Abstract:
This article describes the synthesis of stereolithography (SLA) 3D-printed catalyst-impregnated devices and their evaluation
in the organocatalyzed Friedel–Crafts alkylation of N–Me–indole with trans-β-nitrostyrene. Using a low-cost SLA
3D printer and freeware design software, different devices were designed and 3D-printed using a photopolymerizable resin
containing a thiourea-based organocatalyst. The architectural control offered by the 3D-printing process allows a
straightforward production of devices endowed with different shapes and surface areas, with high reproducibility.
The 3D-printed organocatalytic materials promoted the formation of the desired product up to a 79% yield, although with
longer reaction times compared to reactions under homogeneous conditions
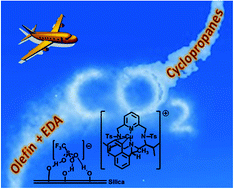
Continuous flow asymmetric cyclopropanation reactions using Cu(I) complexes of Pc-L* ligands supported on silica as catalysts with carbon dioxide as a carrier
B. Castano, E. Gallo, D.J. Cole-Hamilton, V. Dal Santo, R. Psaro, A. Caselli Green Chem., 2014, 16, 3202-3209. [Link]
Abstract:
Continuous flow heterogeneous asymmetric cyclopropanations catalysed by supported hydrogen-bonded (SHB) chiral copper(i) complexes of pyridine containing tetraazamacrocyclic ligands Pc-L*
using CO2 as a transport vector are described. The catalytic system showed high stability and good recyclability without loss of activity for at least 24 h in CO2 and catalyst turnover numbers
up to 440 were obtained with excellent conversion (up to 99%) and high selectivity (up to 88%). No leaching of copper was observed. Cyclopropane products from both aromatic and aliphatic olefins
were obtained in good yields with enantiomeric excesses up to 72%.