Organometallic catalysis and catalysts for sensors
Design and synthesis of organometallic catalysts – Heterogeneization of homogeneous catalysts – Mechanistic studies for process optimization – Catalytic sensors
Indoles from Alkynes and Aryl Azides: Scope and Theoretical Assessment of Ruthenium Porphyrin-Catalyzed Reactions
D. Intrieri, D. M. Carminati, P. Zardi, C. Damiano, G. Manca, E. Gallo, C. Mealli Chem. Eur. J., 2019,16591-16605 [Link]
Abstract:
Symbiotic experimental/computational study analyzed the Ru(TPP)(NAr)2-catalyzed one-pot formation of indoles from alkynes
and aryl azides. Thirty different C3-substituted indoles were synthesized and the best performance, in term of yields and
regioselectivities, was observed when reacting ArC≡CH alkynes with 3,5-(EWG)2C6H3N3 azides, whereas the reaction was less
efficient when using electron-rich aryl azides. A DFT analysis describes the reaction mechanism in terms of the energy costs
and orbital/electronic evolutions; the limited reactivity of electron-rich azides was also justified. In summary, PhC≡CH
alkyne interacts with one NAr imido ligand of Ru(TPP)(NAr)2 to give a residually dangling C(Ph) group, which, by coupling
with a C(H) unit of the N-aryl substituent, forms a 5+6 bicyclic molecule. In the process, two subsequent spin changes allow
inverting the conformation of the sp2 C(Ph) atom and its consequent electrophilic-like attack to the aromatic ring. The
bicycle isomerizes to indole via a two-step outer sphere H migration. Eventually, a ‘Ru(TPP)(NAr)’ mono-imido active catalyst
is reformed after each azide/alkyne reaction.

Improving C=N Bond Reductions with (Cyclopentadienone)iron Complexes: Scope and Limitations
M. Cettolin, X. Bai, D. Lübken, M. Gatti, S. V. Facchini, U. Piarulli, L. Pignataro, C. Gennari Eur. J. Org. Chem., 2019, 647-654 [Link]
Abstract:
Herein, we broaden the application scope of (cyclopentadienone)iron complexes 1 in C=N bond reduction. The catalytic scope of
pre-catalyst 1b, which is more active than the “Knölker complex” (1a) and other members of its family, has been expanded to
the catalytic transfer hydrogenation (CTH) of a wider range of aldimines and ketimines, either pre-isolated or generated in
situ. The kinetics of 1b-promoted CTH of ketimine S1 were assessed, showing a pseudo-first order profile, with TOF = 6.07 h–1
at 50% conversion. Moreover, the chiral complex 1c and its analog 1d were employed in the enantioselective reduction of
ketimines and reductive amination of ketones, giving fair to good yields and moderate enantioselectivity.

Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes
F. Ferretti, D.R. Ramadan, F. Ragaini ChemCatChem, 2019, 4450-4488 [Link]
Abstract:
Nitroarenes are the entry point for the production of most nitrogen-containing aromatic compounds. Thus, any transformation that leads directly
from them to the final product allows saving one or more synthetic steps. This review deals with homogeneously catalyzed
reactions leading to the formation of N-heterocyclic compounds from nitroarenes or nitroalkenes in one pot. Reactions that
lead to the intermediate formation of amines are not considered. Carbon monoxide is the most often employed reductant because
it allows selective reactions, is cheap, and only produces CO2 as stoichiometric byproduct. However, the difficulty in
handling pressurized CO has stimulated in recent years the development of CO-surrogates, that is molecules able to liberate
CO during the reaction. The use of phosphines and diols has also been developed in conjunction with molybdenum catalysts.
The review focusses in more detail on the literature in the period 2006–2018, but reference to earlier work is made when
necessary to put recent results in a more general context.
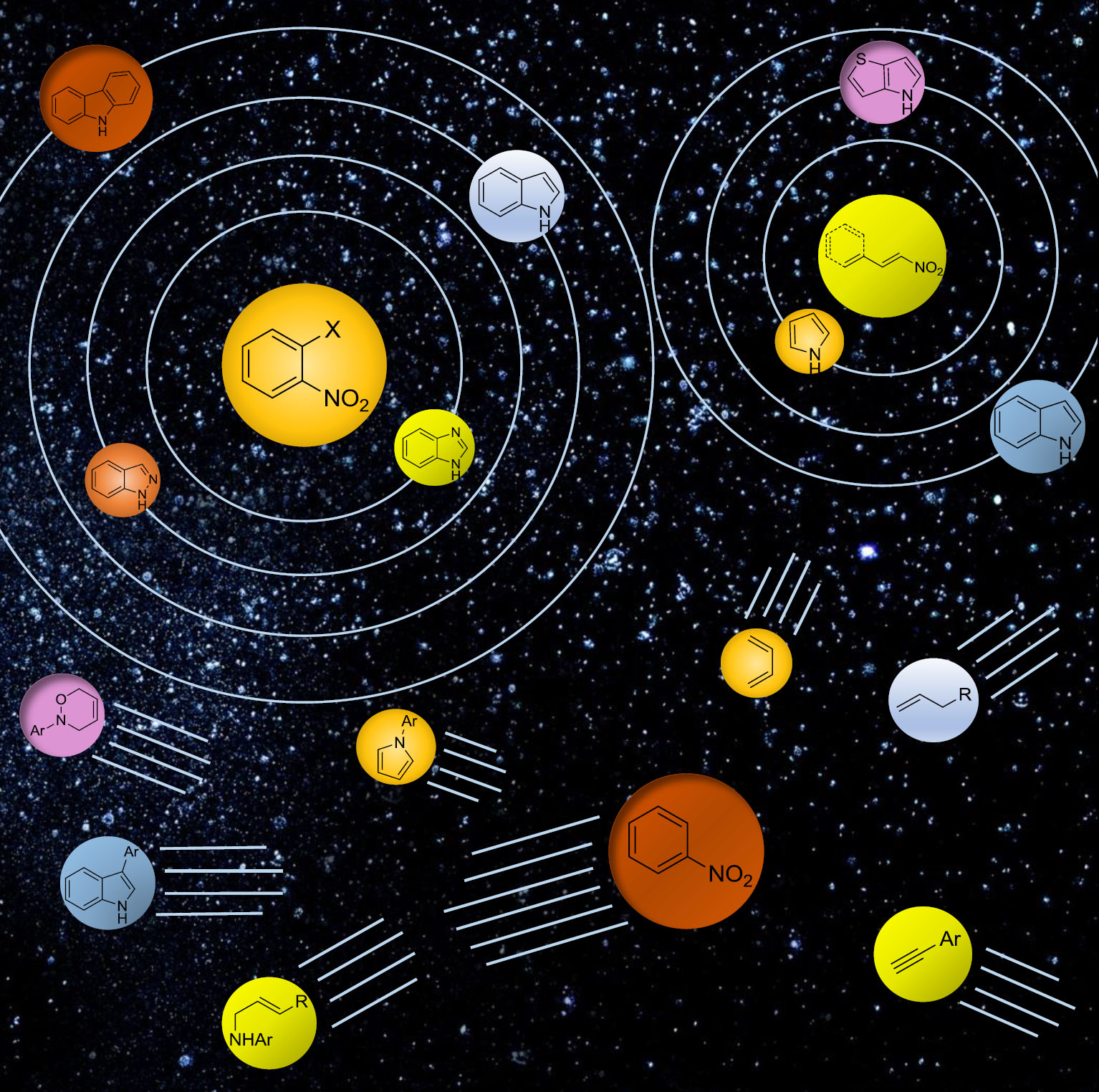
Palladium/iodide catalyzed oxidative carbonylation of aniline to diphenylurea: Effect of ppm amounts of iron salts
F. Ferretti, E. Barraco, C. Gatti, D.R. Ramadan, F. Ragaini Journal of Catalysis, 2019, 257-266 [Link]
Abstract:
The palladium/iodide couple is the most investigated catalytic system for the oxidative carbonylation of amines to give
ureas or carbamates. In reinvestigating it, we found that the most prominent role of iodide is to etch the stainless steel
of the autoclave employed in most of previous works, releasing in solution small amounts of iron salts. The latter are much
better promoters than iodide itself. Iron and iodide have a complex interplay and, depending on relative ratios, can even
deactivate each other. The presence of a halide is beneficial, but chloride is better than iodide in this respect. The ideal
Fe/Pd ratio is around 10, but even an equimolar amount of iron with respect to palladium (0.02 mol% with respect to aniline,
corresponding to 12 ppm Fe with respect to the whole solution) is sufficient to boost the activity of the catalytic system.
Such small amount may also come from Fe(CO)5 impurities present in the CO gas when stored in steel tanks. The role of the
solvent has also been investigated. It was found that the reason for the better selectivity in some cases is at least in
part due to a hydrolysis of the solvent itself, which removes the coproduced water.
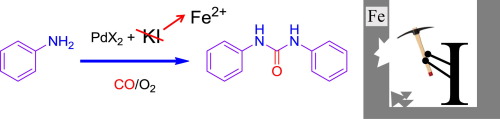
Hydrogen-Borrowing Amination of Secondary Alcohols Promoted by a (Cyclopentadienone)iron Complex
X. Bai, F. Aiolfi, M. Cettolin, U. Piarulli, A. Dal Corso, L. Pignataro, C. Gennari Synthesis, 2019, 51, 3545-3555 [Link]
Abstract:
Thanks to a highly active catalyst, the scope of the (cyclopentadienone)iron complex-promoted ‘hydrogen-borrowing’ (HB)
amination has been expanded to secondary alcohols, which had previously been reported to react only in the presence of large
amounts of co-catalysts. A range of cyclic and acyclic secondary alcohols were reacted with aromatic and aliphatic amines
giving fair to excellent yields of the substitution products. The catalyst was also able to promote the cyclization of diols
bearing a secondary alcohol group with primary amines to generate saturated N-heterocycles.
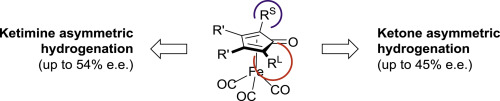
Chiral (cyclopentadienone)iron complexes with a stereogenic plane as pre-catalysts for the asymmetric hydrogenation of polar double bonds
X. Bai, M. Cettolin, G. Mazzoccanti, M. Pierini, U. Piarulli, V. Colombo, A. Dal Corso, L. Pignataro, C. Gennari Tetrahedron, 2019, 75, 1415-1424 [Link]
Abstract:
In this paper, we describe a small library of easy-to-prepare chiral (cyclopentadienone)iron pre-catalysts for enantioselective
CO and CN hydrogenations. Starting from readily accessible achiral materials, six chiral (cyclopentadienone)iron complexes
(1a-f) possessing a stereogenic plane were synthesized in racemic form. Based on the screening of pre-catalysts (±)-1a-f
in the hydrogenation of ketones and ketimines, we selected two complexes (1a and 1d) for resolution by semipreparative
enantioselective HPLC. The absolute configuration of the separated enantiomers of 1a and 1d was assigned by XRD analysis
(1a) and by comparison between experimental and DFT-calculated ECD and ORD spectra (1d). The enantiopure pre-catalysts
(S)-1a and (R)-1d were tested in the asymmetric hydrogenation of several ketones and ketimines and showed good activity and
modest enantioselectivity, the e.e. values ranging from very low to moderate (54%).
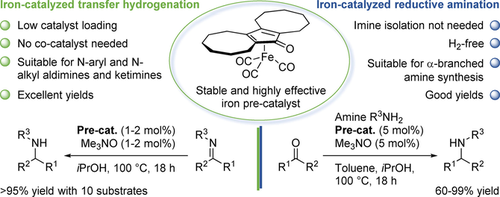
Efficient Synthesis of Amines by Iron-Catalyzed C=N Transfer Hydrogenation and C=O Reductive Amination
S. Vailati Facchini, M. Cettolin, X. Bai, G. Casamassima, L. Pignataro, C. Gennari, U. Piarulli, Adv. Synth. Catal., 2019, 360, 1054-1059 [Link]
Abstract:
Here we report the catalytic transfer hydrogenation (CTH) of non-activated imines promoted by a Fe-catalyst in the absence
of Lewis acid co-catalysts. Use of the (cyclopentadienone)iron complex 1, which is much more active than the classical
‘Knölker complex’ 2, allowed to reduce a number of N-aryl and N-alkyl imines in very good yields using iPrOH as hydrogen
source. The reaction proceeds with relatively low catalyst loading (0.5–2 mol%) and, remarkably, its scope includes also
ketimines, whose reduction with a Fe-complex as the only catalyst has little precedents. Based on this methodology, we
developed a one-pot CTH protocol for the reductive amination of aldehydes/ketones, which provides access to secondary amines
in high yield without the need to isolate imine intermediates.
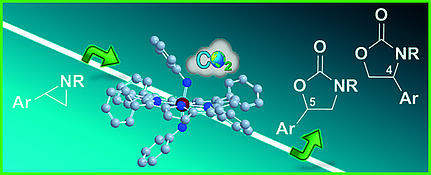
Ruthenium Porphyrin Catalyzed Synthesis of Oxazolidin-2-ones by Cycloaddition of CO2 to Aziridines
D. Carminati, E. Gallo, C. Damiano, A. Caselli, D. Intrieri Eur. J. Inorg. Chem., 2018, 5258-5262 [Link]
Abstract:
The reaction between N-substituted-2-arylaziridines and CO2 is efficiently promoted by ruthenium(VI) imidoporphyrin
complexes and yields a mixture of 5-aryl (A) and 4-aryl (B) substituted oxazolidin-2-ones with a regioisomeric A/B ratio
up to 99:1. Several oxazolidin-2-one molecules were synthesized at 100 °C and 0.6 MPa of carbon dioxide by using the low
catalytic loading of 1 mol%. The formation of a deactivated compound, deriving from the ruthenium catalyst, suggested a
possible catalytic role of imido nitrogen atoms.
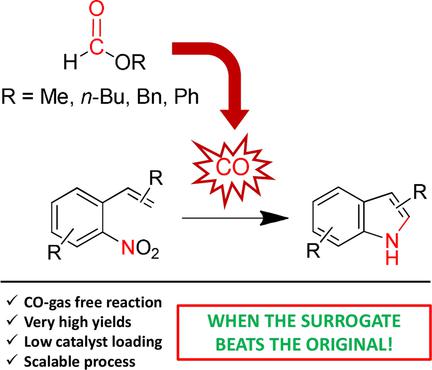
Synthesis of N-Heterocycles by Reductive Cyclization of Nitro Compounds using Formate Esters as Carbon Monoxide Surrogates
D. Formenti, F. Ferretti, F. Ragaini, ChemCatChem, 2018, 10, 148-152 [Link]
Abstract:
A series of alkyl and aryl formate esters were evaluated as CO sources in the Pd- and Pd/Ru-catalyzed reductive cyclization
of substituted nitro compounds to afford heterocycles, especially indoles. Phenyl formate was found to be the most effective,
and it allowed the desired products to be obtained in excellent yields, often higher than those previously reported with
pressurized CO. Through detailed experiments and kinetic studies, we were able to discern between metal-catalyzed and
base-mediated activation of phenyl formate and confirmed that just the base was effective in catalyzing its decarbonylation.
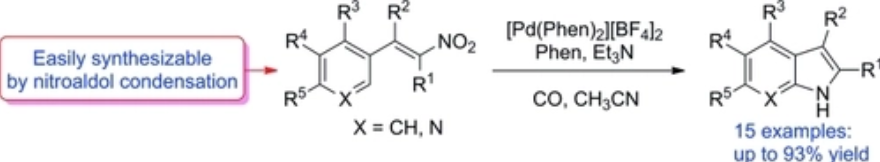
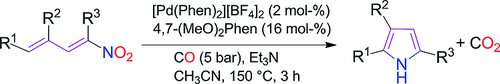
A Synthetic Methodology for Pyrroles from Nitrodienes
M.A. EL-Atawy, F. Ferretti, F. Ragaini, Eur J Org Chem , 2018, 34, 4818-4825 [Link]
Abstract:
Palladium complexes containing the ligand 4,7-dimethoxy-1,10-phenanthroline have been used to catalyze the reductive
cyclization of nitrodienes using carbon monoxide as the reductant to give pyrroles. Carbon dioxide was the only
stoichiometric byproduct of the reaction. The yields were good and the starting materials can be easily synthesized in two
steps by a cross-aldol condensation reaction followed by a Henry reaction. Different substitution patterns are tolerated by
this novel synthetic strategy.
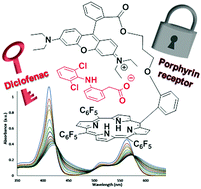
Sensing of diclofenac by a porphyrin-based artificial receptor
D. Intrieri, C. Damiano, S. Rizzato, R. Paolesse, M. Venanzi, D. Monti, M. Savioli, M. Stefanelli E. Gallo New J. Chem., 2018, 42, 15778-15783 [Link]
Abstract:
The reported studies deal with the synthesis of porphyrin chemosensor 2, designed for the detection of the emerging pollutant diclofenac.
Owing to the peculiar structure of its molecular frame, which is composed of a tetrapyrrolic platform linked to a rhodamine B residue,
receptor 2 reversibly interacts with diclofenac sodium salt (DCF)Na. The resulting 2@DCF adduct was detected by UV-Vis spectroscopy in
a large pH range (5.5–9.0) as well as in the presence of competitive analytes. Both static and timeresolved fluorescence, resonance
light scattering (RLS) and UV-Vis spectroscopies allowed for the evaluation of the binding behaviour, in terms of the association constant
and structural features of the formed 2@DCF. In particular, the host–guest recognition event occurs with the growth of large porphyrin aggregates,
as stated by the quenching of the fluorescence emission as well as the enhancement of RLS intensities, and with an overall 1: 1 binding constant of
about 105 M-1.
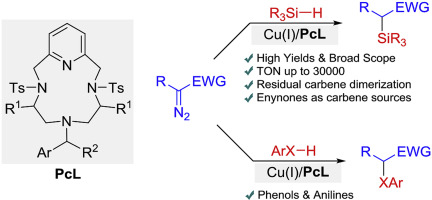
Carbene X-H bond insertions catalyzed by copper(I) macrocyclic pyridine-containing ligand (PcL) complexes.
G. Tseberlidis, A. Caselli, R. Vicente J. Organomet. Chem., 2017, 835, 1-5. [Link]
Abstract:
Cyclopentadienone-based iron complexes were used for the first time to successfully catalyze the diastereoselective hydrogenation
of enantiopure imines. Chiral amines, including valuable biologically active products, were obtained often as enantiomerically
pure compounds. Computational studies helped to elucidate the chemical and stereochemical aspects of the iron-catalyzed reaction.
Catalytic Applications of Pyridine-Containing Macrocyclic Complexes.
G. Tseberlidis, D. Intrieri, A. Caselli. Eur. J. Inorg. Chem, 2017, 3589-3603. [Link]
Abstract:
The introduction of a pyridine moiety into the skeleton of a polyazamacrocyclic ligand affects both the thermodynamic properties and the coordination kinetics of the resulting metal complexes.
These features have attracted great interest from the scientific community in recent years. The field of application of pyridine‐containing macrocyclic ligands ranges from biology to supramolecular
chemistry, encompassing MRI, molecular recognition, materials and catalysis. In this microreview we provide a perspective of the catalytic applications of metal complexes of pyridine‐containing
macrocycles, including an account of investigations from the authors' laboratories dealing with stereoselective C–C and C–O bond‐forming reactions. The increased conformational rigidity imposed
by the pyridine ring allowed for the isolation and characterisation of metal complexes in high oxidation states and the study of their relevance in oxidation reactions. On the other hand, the very
different conformations accessible upon the metal coordination and the easily tuneable synthesis of the macrocyclic ligands have been exploited in stereoselective synthesis.
Iron catalyzed diastereoselective hydrogenation of chiral imines.
D. Brenna, S. Rossi, F. Cozzi, M. Benaglia Org. Biomol. Chem, 2017, 15, 5685-5688. [Link]
Abstract:
Cyclopentadienone-based iron complexes were used for the first time to successfully catalyze the diastereoselective hydrogenation
of enantiopure imines. Chiral amines, including valuable biologically active products, were obtained often as enantiomerically
pure compounds. Computational studies helped to elucidate the chemical and stereochemical aspects of the iron-catalyzed reaction.
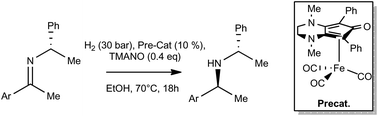
Synthesis of [Bis(hexamethylene)cyclopentadienone]iron Tricarbonyl and its Application to the Catalytic Reduction of C=O Bonds
S. Vailati Facchini, J.-M. Neudörfl, L. Pignataro, M. Cettolin, C. Gennari, A. Berkessel, U. Piarulli Chemcatchem, 2017, 9, 1461-1468. [Link]
Abstract:
Herein, we report the synthesis of [bis(hexamethylene)cyclopentadienone]iron tricarbonyl (1b) by the reaction of cyclooctyne with
Fe(CO)5 and the investigation of its catalytic properties in C=O bond reduction. As a result of the peculiar reactivity of cyclooctyne,
1b was formed in good yield (56%) by intermolecular cyclative carbonylation/complexation with Fe(CO)5. Compound 1b was characterized fully
and its crystal structure was determined by using XRD. Catalytic tests revealed that, upon in situ activation with Me3NO, 1b promotes the
hydrogenation of ketones, aldehydes, and activated esters as well as the transfer hydrogenation of ketones and shows a higher activity than
the classical “Knölker complex” (1a). Studies on the hydrogenation kinetics in the presence of 1a and 1b (respectively) suggest that this
difference in activity is probably caused by the better stability of the 1b-derived complex than that of the in situ generated Knölker–Casey
catalyst.
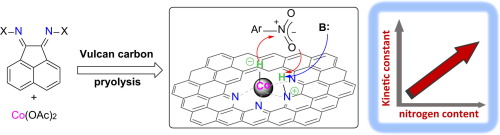
Co-based heterogeneous catalysts from well-defined α-diimine complexes: Discussing the role of nitrogen
D. Formenti, F. Francesco, C. Topf, A-E. Surkus, M-M Pohl, J. Radnik, M. Schneider, K. Junge, M. Beller, F. Ragaini Journal of Catalysis, 2017, 351, 79-89. [Link]
Abstract:
Ar-BIANs and related α-diimine Co complexes were wet impregnated onto Vulcan XC 72 R carbon black powder and used as precursors
for the synthesis of heterogeneous supported nanoscale catalysts by pyrolysis under argon at 800 °C.
The catalytic materials feature a core-shell structure composed of metallic Co and Co oxides decorated with nitrogen-doped
graphitic layers (NGr). These catalysts display high activity in the liquid phase hydrogenation of aromatic nitro compounds
(110 °C, 50 bar H2) to give chemoselectively substituted aryl amines. The catalytic activity is closely related to the amount
and type of nitrogen atoms in the final catalytic material, which suggests a heterolytic activation of dihydrogen.
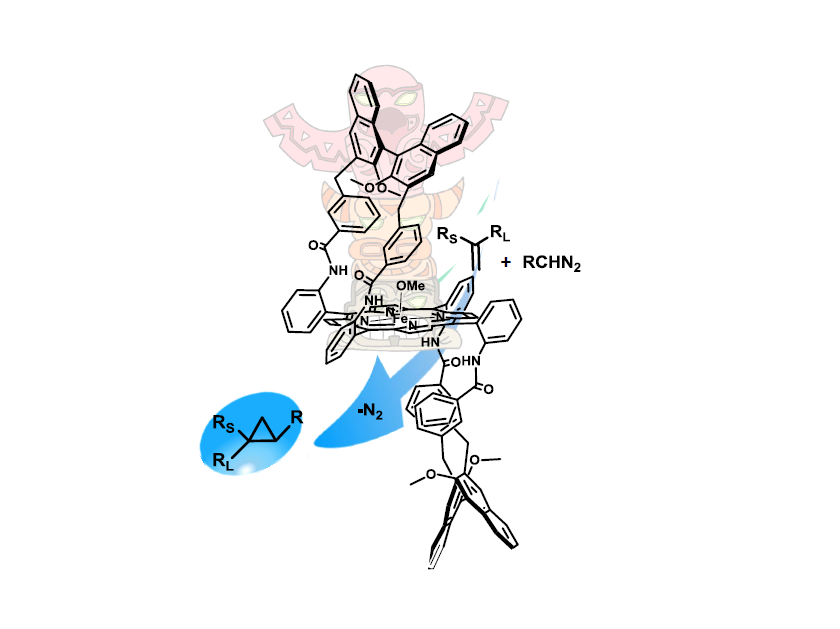
Synthesis, Characterisation and Catalytic Use of Iron Porphyrin Amino Ester Conjugates.
D. M. Carminati, D. Intrieri, S. Le Gac, T. Roisnel, B. Boitrel, L. Toma, L. Legnani, E. Gallo, New J. Chem., 2017, 41, 5950-5959 [Link]
Abstract:
The present study deals with the synthesis and characterisation of C2-symmetrical chiral Fe(III)(porphyrin)(OMe) catalysts; these catalysts display a totem
structure, where each section is assigned to have a specific catalytic activity. The chiral portions of the porphyrin ligand are constituted by amino acid
residues, which form a chiral cavity by surrounding both faces of the porphyrin plane, as clearly displayed by the X-ray structure
of a free-base porphyrin. The Fe(III)(porphyrin)(OMe)-catalysed cyclopropanation of a-methylstyrene by diazo compounds occurred
with excellent diastereoselectivities and a modest enantiocontrol. Thus, the obtained catalytic results have been rationalised by
performing a DFT investigation and will be fundamental in the future modification of the molecular structure of chiral ligands to
improve their catalytic performance.
Designing ‘Totem’ C2-Symmetrical Iron Porphyrin Catalysts for Stereoselective Cyclopropanations
D. M. Carminati, D. Intrieri, A. Caselli, S. Le Gac, B. Boitrel, L. Toma, L. Legnani E. Gallo Chem. Eur. J., 2016, 22, 13599-13612. [Link]
Abstract:
The catalytic activity of the iron(III) C2 chiral porphyrin Fe(2)(OMe) in the alkene cyclopropanation is herein reported. The catalyst promoted the reaction of
differently substituted styrenes with diazo derivatives with trans-diastereoselectivities and enantioselectivities up to 99:1 and 87%, respectively. In addition,
high TON and TOF values (up to 10 000 and 120 000 h-1, respectively) were observed indicating good activity and stability of the
catalyst in optimised experimental conditions. The study of the cyclopropanation reaction revealed that the porphyrin skeleton
is composed of two ‘totem’ parts which were independently responsible for the observed enantio- and diastereoselectivities. To
further our research we also investigated the catalytic role of the methoxy axial ligand coordinated to the iron atom. The
molecular structure of Fe(2)(OMe) was optimised by DFT calculations which were also employed to achieve mechanistic details of
the carbene transfer reaction.
Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of β-Nitrostyrenes with Carbon Monoxide as the Reductant
F. Ferretti, M.A. El-Atawy, S. Muto, M. Hagar, E. Gallo, F Ragaini, Eur J Org Chem, 2015, 26, 5712-5715. [Link]
Abstract:
An efficient catalytic cyclization of -nitrostyrenes to indoles was developed. The reaction was applied to the synthesis
of 3-arylindoles and 2-alkylindoles. Given that in the latter case the starting nitrostyrenes can be easily obtained by a
Henry reaction, the present method allows indoles to be obtained in a two-step sequence starting from cheap reactants.